Atom: Unlocking Atomic Secrets
Quantum theory and the atom are key concepts in modern physics. They explain the behavior of particles at the smallest scales.
Understanding quantum theory can seem complex. But, it helps us explain how atoms work, which are the building blocks of everything around us. Quantum theory explores the strange and fascinating world of the very small. This field has led to many important discoveries and technologies.
From the structure of atoms to the weird behavior of particles, quantum theory changes how we see the universe. In this blog, we will dive into the basics of quantum theory and its connection to atoms. Get ready for a journey into the tiny, yet incredibly important, world of quantum physics.
Credit: chem.libretexts.org
Introduction To Quantum Theory
Quantum theory is a fundamental branch of physics. It explains the nature and behavior of matter and energy. At the smallest scales, it provides insights into the atomic world. This theory challenges classical physics. It reveals complex and fascinating phenomena.
Historical Background
The roots of quantum theory trace back to the early 20th century. Scientists like Max Planck and Albert Einstein laid its foundation. Planck introduced the idea of quantized energy levels. Einstein’s work on the photoelectric effect further supported quantum ideas. These early breakthroughs changed the understanding of atoms. They opened new paths in science and technology.
Key Principles
Several key principles define quantum theory. One is the concept of wave-particle duality. It suggests particles like electrons have both wave and particle characteristics. Another principle is the uncertainty principle by Heisenberg. It states that certain properties cannot be measured precisely at the same time. Quantum entanglement is another fascinating aspect. It shows particles can be connected over vast distances. These principles challenge our classical view of reality. They offer a deeper understanding of the universe.

Credit: www.britannica.com
The Atomic Model
The atomic model helps explain how matter is built. It describes the structure of atoms, the building blocks of all things. The model has changed over time, reflecting new discoveries and ideas. Understanding it is key to grasping the basics of chemistry and physics.
Evolution Of Atomic Models
The concept of the atom began with ancient philosophers. They thought matter was made of tiny, indivisible particles. In the early 1800s, John Dalton proposed a more scientific atomic theory. He said atoms were solid spheres, each element having its own kind.
Later, J.J. Thomson discovered the electron. This led to the “plum pudding” model. He imagined atoms as balls of positive charge with electrons scattered inside. Then came Ernest Rutherford’s gold foil experiment. It showed that atoms have a small, dense nucleus. This led to a new model where electrons orbit the nucleus.
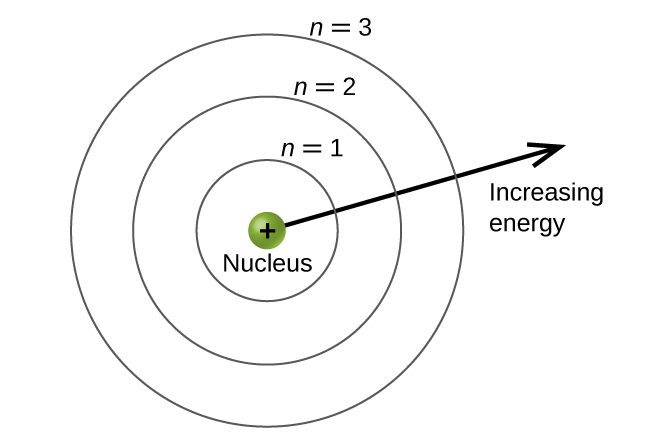
Bohr’s Contribution
Niels Bohr made a big impact in 1913. He suggested electrons orbit the nucleus in fixed paths. These paths are called energy levels. His model explained why atoms emit light in certain colors.
Bohr’s model was simple but powerful. It introduced the idea of quantized energy levels. This means electrons can only exist at certain distances from the nucleus. This concept was a stepping stone to modern quantum theory.
Wave-particle Duality
Quantum theory has transformed our understanding of the universe. One of its key concepts is wave-particle duality. This principle suggests that every particle or quantum entity can exhibit both wave and particle properties. Let’s delve deeper into this fascinating idea.
Light As Waves And Particles
Light behaves both as a wave and as a particle. In some experiments, light acts like a wave, showing properties like interference and diffraction. In other experiments, light acts like a particle, with photons hitting a surface one by one.
Think of light as a wave when it travels. Imagine ripples on a pond. But also, consider light as a stream of particles. Like a spray of tiny bullets. This dual nature puzzled scientists for years. The famous double-slit experiment showed light behaving as both.
Implications For Atoms
Wave-particle duality also applies to atoms. Electrons around an atom’s nucleus exhibit wave-like properties. They don’t orbit the nucleus like planets around the sun. Instead, they exist in cloud-like regions called orbitals.
These orbitals are not fixed paths. They’re probability zones where an electron is likely to be found. This concept changes our view of atomic structure. It means atoms are not solid, but rather fuzzy and dynamic. Understanding this duality helps in many fields, from chemistry to nanotechnology.

Credit: www.britannica.com
Quantum Mechanics And The Atom
Quantum mechanics is the fascinating study of how particles, like electrons, behave on an incredibly small scale. This branch of physics explains the strange, yet fundamental, ways in which atoms and their components interact. You might wonder how these tiny particles can lead to the vast complexities of the universe. Well, let’s take a closer look at how quantum mechanics unravels the mysteries of the atom.
Schrodinger Equation
The Schrodinger Equation is a cornerstone of quantum mechanics. It describes how the quantum state of a physical system changes over time. Think of it as a mathematical tool that helps predict where an electron might be around a nucleus.
Imagine trying to track a buzzing fly in a room. You can’t pinpoint its exact location at any given moment, but you can estimate the regions it frequents the most. Similarly, the Schrodinger Equation helps scientists understand the probable locations of electrons around an atom’s nucleus. It’s like having a superpower that lets you see the invisible paths of these tiny particles.
Quantum States
Quantum states are the different possible conditions an atom can exist in. Each state represents a unique set of properties, like energy levels and electron configurations. It’s like having a wardrobe full of different outfits, each suitable for a specific occasion.
What if I told you that atoms can be in multiple states at once? This is called superposition. It challenges our everyday understanding of reality, where things are either here or there, but not both. Quantum states open a world of possibilities, influencing technologies like quantum computing, which promises to revolutionize how we process information.
Have you ever wondered how your smartphone works so efficiently? Quantum mechanics and the study of atoms are key to the development of semiconductors, which power your electronic devices. As you explore the quantum realm, think about how these tiny particles shape the technology you use daily.
Quantum theory and its understanding of the atom may seem abstract, but they have practical implications. They push the boundaries of what we know and drive innovation. So, next time you use your phone or computer, remember the quantum dance of atoms that makes it all possible.
Uncertainty Principle
Quantum Theory explores the mysterious world of atoms. The Uncertainty Principle reveals that we cannot know both the position and momentum of a particle precisely. This principle challenges how we understand the smallest parts of the universe.
Quantum mechanics introduces a fascinating concept known as the Uncertainty Principle, a core element that challenges our understanding of atomic behavior. This principle suggests that there are limits to how precisely we can measure certain pairs of physical properties simultaneously. Think of it as trying to pinpoint the exact position and speed of a tiny electron at the same time. It’s like attempting to catch a fleeting shadow—no matter how hard you try, it always seems to slip through your fingers.
Heisenberg’s Insight
Werner Heisenberg, a pivotal figure in quantum mechanics, formulated the Uncertainty Principle. His insight was groundbreaking. He realized that the act of measuring one property affects the other. If you measure an electron’s position precisely, its velocity becomes uncertain. This idea overturns the classical notion that every aspect of the universe is predictable.
Heisenberg’s insight is not just a theoretical concept; it has practical implications. Consider the technology you use daily. Quantum mechanics, influenced by the Uncertainty Principle, is foundational to semiconductor devices like your smartphone. It shapes the future of computing and communication.
Impact On Atomic Structure
The Uncertainty Principle profoundly influences our understanding of atomic structure. Atoms, the building blocks of matter, don’t behave as solid entities. Their electrons exist in a cloud of probability rather than fixed orbits. This principle helps explain why atoms are stable despite their electrons’ constant motion.
Imagine trying to predict the exact location of an electron in an atom. You can’t pin it down. Instead, you can only calculate probabilities of where it might be. This uncertainty ensures that atoms don’t collapse into themselves, maintaining their integrity.
Now, think about your own experiences with uncertainty. Have you ever faced situations where outcomes seemed unpredictable? Just like atoms, embracing uncertainty in life can lead to stability and growth. The Uncertainty Principle teaches us that not knowing everything can be beneficial.
Is uncertainty a challenge or an opportunity for you? Consider how this principle might change your perspective on problem-solving or decision-making. As you explore this concept, keep in mind that uncertainty can drive innovation and discovery, just as it does in the quantum world.
Quantum Tunneling
Quantum tunneling defies classical physics. In this phenomenon, particles pass through barriers. These barriers are usually impenetrable. The process occurs due to the wave-like nature of particles. Quantum mechanics explains this behavior. It’s a key concept in understanding atoms and subatomic particles.
Phenomenon Description
In classical physics, particles need energy to overcome barriers. Quantum tunneling changes this notion. Particles behave like waves in this context. They spread out and overlap the barrier. This overlap gives a probability of passing through. The barrier doesn’t stop them completely. The wave function of the particle extends beyond the barrier. This extension allows the particle to appear on the other side. Despite the lack of sufficient energy, it passes through. This unique behavior is crucial in quantum theory.
Applications In Technology
Quantum tunneling has practical uses. It plays a role in modern technology. Tunneling is essential in semiconductor devices. It helps in the function of tunnel diodes. These diodes conduct current more efficiently. They are used in high-speed electronics. Tunneling also contributes to nuclear fusion. It allows particles to merge at lower energies. This process is important for energy production. Quantum tunneling is crucial in scanning tunneling microscopes. These devices image surfaces at the atomic level. They provide detailed views of atomic structures. This insight aids in materials science and nanotechnology.
Quantum Entanglement
Quantum entanglement is a fascinating concept in quantum theory. It involves particles that become interconnected, behaving as one, even when separated by vast distances. This phenomenon challenges our understanding of the universe. Many find it both perplexing and intriguing. It suggests that the properties of one particle can instantly affect another. This happens no matter how far apart they are. Scientists continue to explore its mysteries, seeking answers and applications.
Entangled Particles
Entangled particles share a unique connection. Changing one affects the other. This happens instantly, defying classical physics. Imagine two particles, miles apart. Altering one particle changes its partner. This occurs without delay. It’s as if they communicate faster than light. This concept baffles many. It raises questions about the nature of reality. Physicists study these particles to understand quantum mechanics better.
Implications For Communication
Quantum entanglement offers exciting possibilities for communication. It could lead to more secure channels. Imagine a network where eavesdropping is impossible. Quantum communication promises such security. Entangled particles could transmit information instantly. This might change how we connect across distances. Researchers are exploring these potential applications. They hope to harness this power for future technologies. The dream is faster, safer communication.
Future Of Quantum Theory
Quantum theory is a cornerstone of modern physics. It describes how atoms behave. The future of quantum theory holds promising developments. Researchers continue to explore its depths. New insights could lead to groundbreaking discoveries. These advancements may transform technology and science. Understanding the atom through quantum theory remains crucial. It helps in predicting atomic behaviors. This knowledge can pave the way for future innovations.
Research Directions
Scientists are focusing on quantum computing. They aim to enhance computational power. Quantum computers can solve complex problems quickly. Research also explores quantum entanglement. This phenomenon links particles over vast distances. It could improve communication technologies. Another area is quantum sensing. This involves detecting changes at atomic levels. Enhanced sensors could lead to better imaging techniques. Researchers study quantum cryptography too. It promises secure communication methods.
Potential Technological Advances
Quantum theory may impact energy sectors. It could lead to efficient energy storage. This means more sustainable energy solutions. Medical technology might also benefit. Quantum sensors can improve diagnostics. They offer precise measurements at atomic scales. Quantum computing may optimize drug development. Faster computations can accelerate research processes. In telecommunications, quantum theory could enhance data transmission. It ensures faster and more reliable communication.
Frequently Asked Questions
What Is The Quantum Model Of The Atom?
The quantum model of the atom describes electrons in terms of probabilities. Electrons exist in orbitals around the nucleus. This model replaces fixed orbits with regions where electrons are likely found. It incorporates principles of quantum mechanics.
Who Created The Quantum Theory Model Of The Atom?
Niels Bohr created the quantum theory model of the atom. His model combined classical and quantum concepts. This theory revolutionized our understanding of atomic structure. Bohr’s work laid the foundation for modern quantum mechanics.
Who Applied Quantum Theory To Atoms?
Niels Bohr applied quantum theory to atoms. His model explained electron orbits and energy levels. This groundbreaking work revolutionized atomic physics and laid the foundation for modern quantum mechanics. Bohr’s atomic model significantly advanced our understanding of atomic structure.
What Does Quantum Mean In Atoms?
Quantum in atoms refers to discrete energy levels. Electrons exist in specific orbits, not in between. This principle defines atomic structure.
Conclusion
Quantum theory helps us understand the atom’s structure better. It explains atomic behavior in detail. Scientists continue to make discoveries using quantum theory. This knowledge impacts many fields, such as chemistry and physics. Understanding atoms can lead to new technologies.
Quantum theory remains a key area of study. Keep exploring and learning about this fascinating topic.








